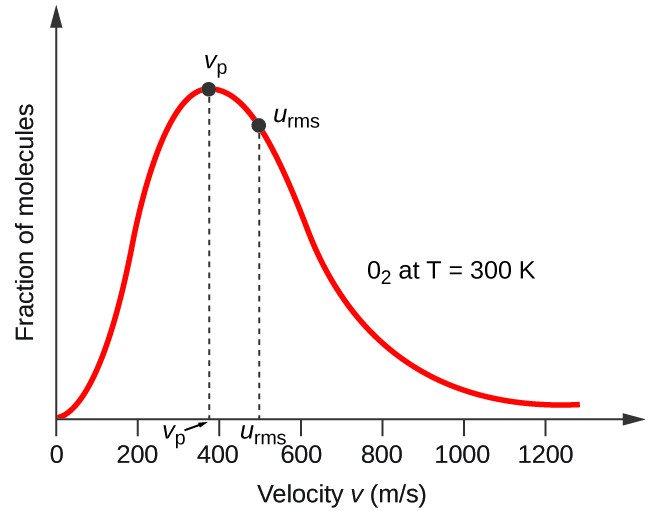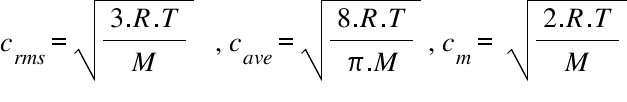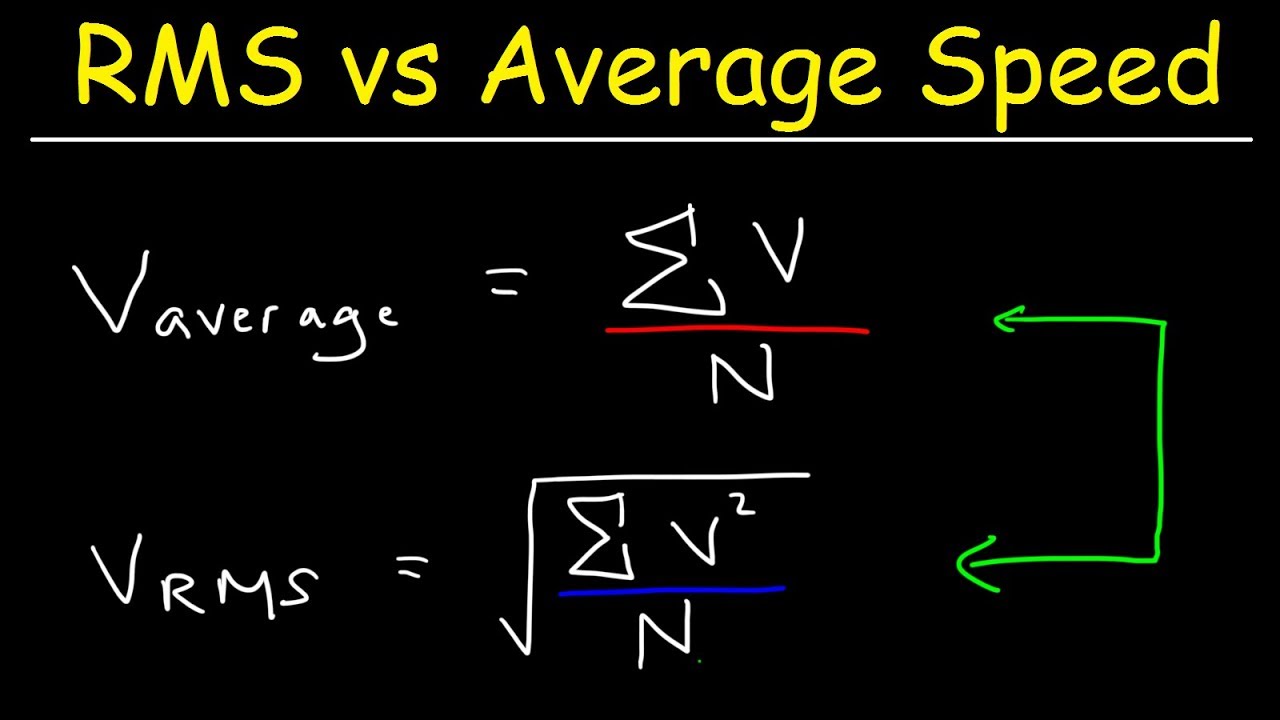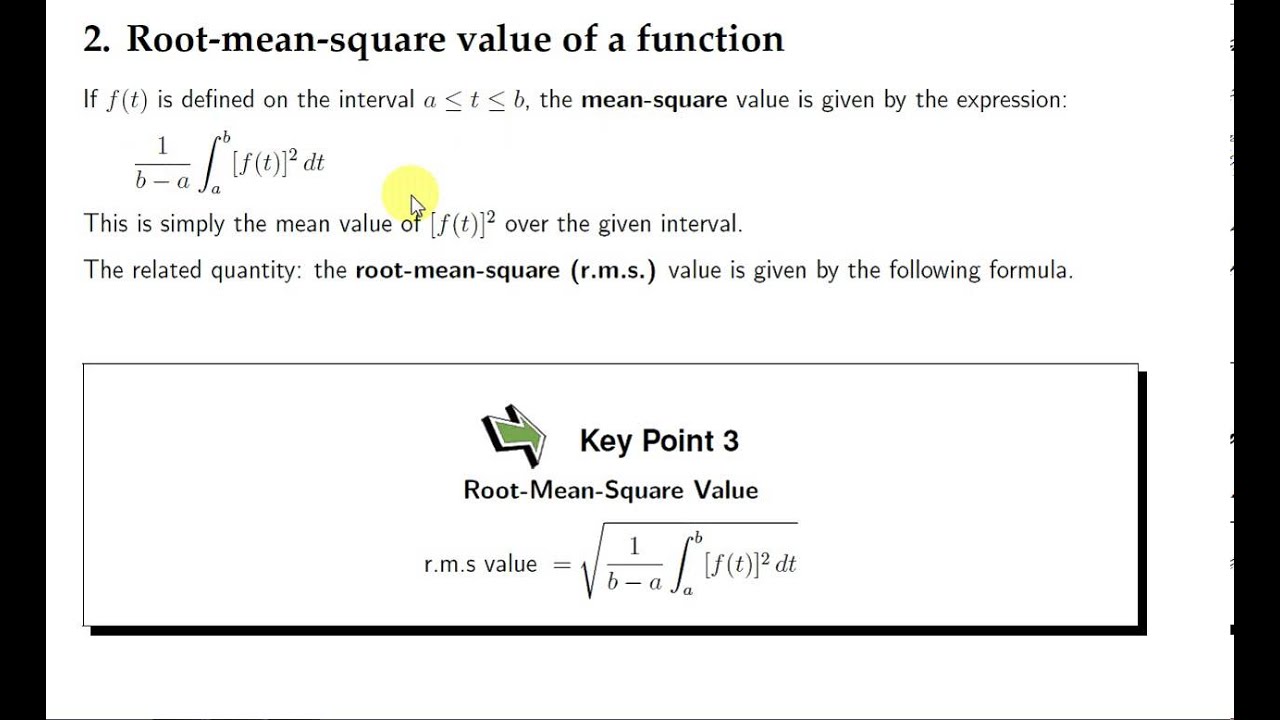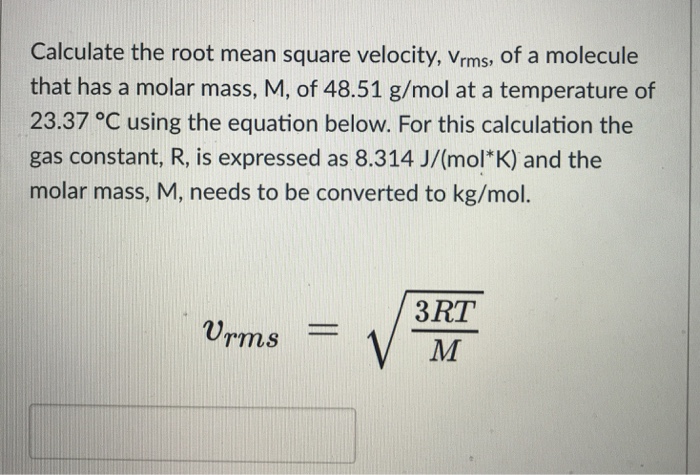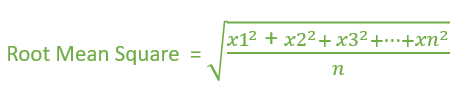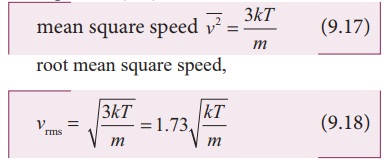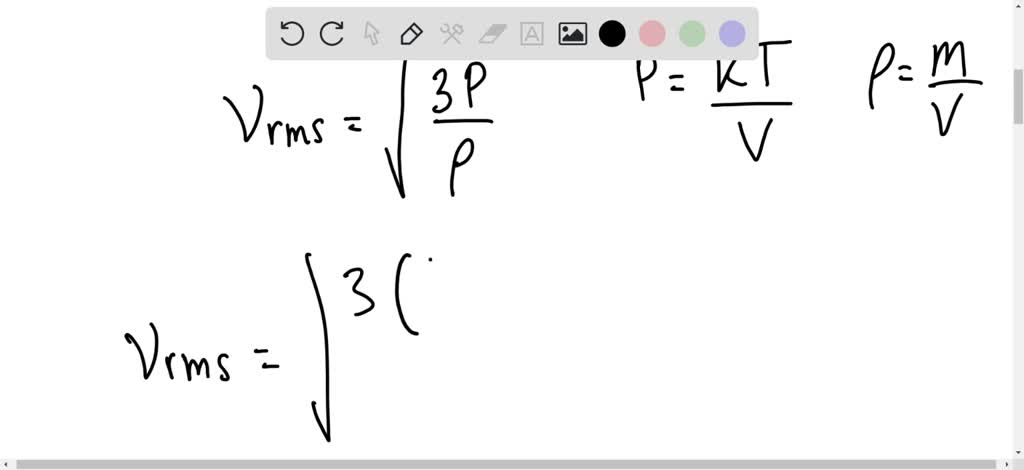
SOLVED: Calculate the root mean square velocity of ammonia (NH3) molecules at 25°C. The molar mass of NH3 is 17.02 g/mol and 1 J = 1 kg m2/s2

How to Calculate the Root Mean Square Speed of Molecules in Gas at a Certain Temperature | Physics | Study.com

RMS Value, Average Value, Peak Value, Peak Factor, Form Factor in AC | Rms, Engineering notes, Electrical circuit diagram